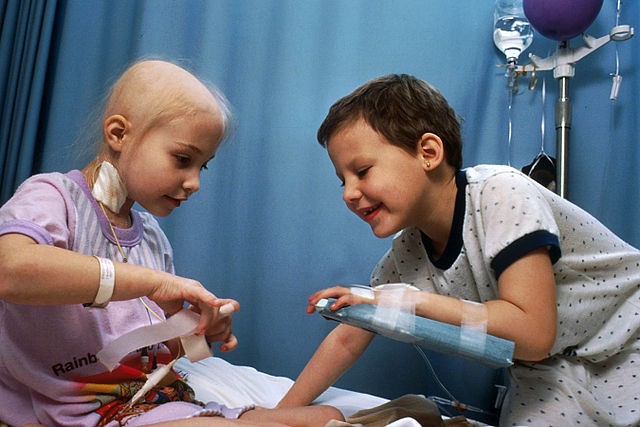
Results of a study published this week in the medical journal Pediatrics said that cannabinoids could have potential benefits for children with seizures, as well as young children experiencing nausea due to chemotherapy treatment.
Meta-analysis of 22 relevant studies on adolescents and children provided data for the study. Findings showed that THC had a beneficial effect for treating nausea in young patients. Cannabidiol, better known as cannabinoid compound CBD, was shown to be effective in treating children suffering from seizures.
Researchers also reported that their research was inconclusive in finding a benefit from cannabis treatment for spasticity, neuropathic pain, post traumatic stress disorder, or Tourette’s syndrome in young patients.
They concluded that more research would be required to determine the role of medical cannabinoids for children. They noted that research was also necessary, in order to further evaluate the “potential psychiatric and neurocognitive adverse effects identified from studies of recreational cannabis use,” and in light of increased legalization.
Despite substantiated findings that cannabinoid compounds may have medicinal benefits, cannabis continues to be recognized as a federal Schedule 1 drug, which defines it as having “no currently accepted medical treatment use in the U.S.,” and puts it in the same category as heroin, GHB, and bath salts. Schedule 1 status also impedes any cannabis research that has not been approved by federal agencies, as well as the National Institute on Drug Abuse (NIDA), which is the biggest public funder of cannabis research in the U.S. Currently, the NIDA is contracted exclusively with the University of Mississippi, to conduct approved cannabis research.
In August of this year, the Drug Enforcement Agency announced that it would not change the status of cannabis as a Schedule 1 drug, in response to petitions that had requested reconsideration for cannabis.
“The DEA and the FDA continue to believe that scientifically valid and well-controlled clinical trials conducted under investigational new drug (IND) applications are the most appropriate way to conduct research on the medicinal uses of marijuana,” the DEA said in a statement at the time.
In the same announcement, the DEA said it would also loosen restrictions and allow additional entities to register with the DEA, to grow and distribute cannabis for use in federally approved research.